EU - Approved Amendment of Annexes I and III to Regulation (EU) No 10/2011 on Plastic Food Contact Materials and Articles
The European Commission has published Regulation (EU) 2019/37 to amend the Union list of authorised substances under Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food. The regulation shall be effective from 31 January 2019. Plastic materials and articles complying with Regulation (EU) No 10/2011 as applicable before the entry into force of this Regulation may be placed on the market until 31 January 2020 and may remain on the market until exhaustion of stocks.
Here are the highlights:
A. Annex I Substances
1. Table 1. “Union list of authorised monomers, other starting substances, macromolecules obtained from microbial fermentation, additives and polymer production aids” is amended as follows:
a) the entries concerning FCM substances No 467, 744, 1066, and 1068 are replaced by the following:
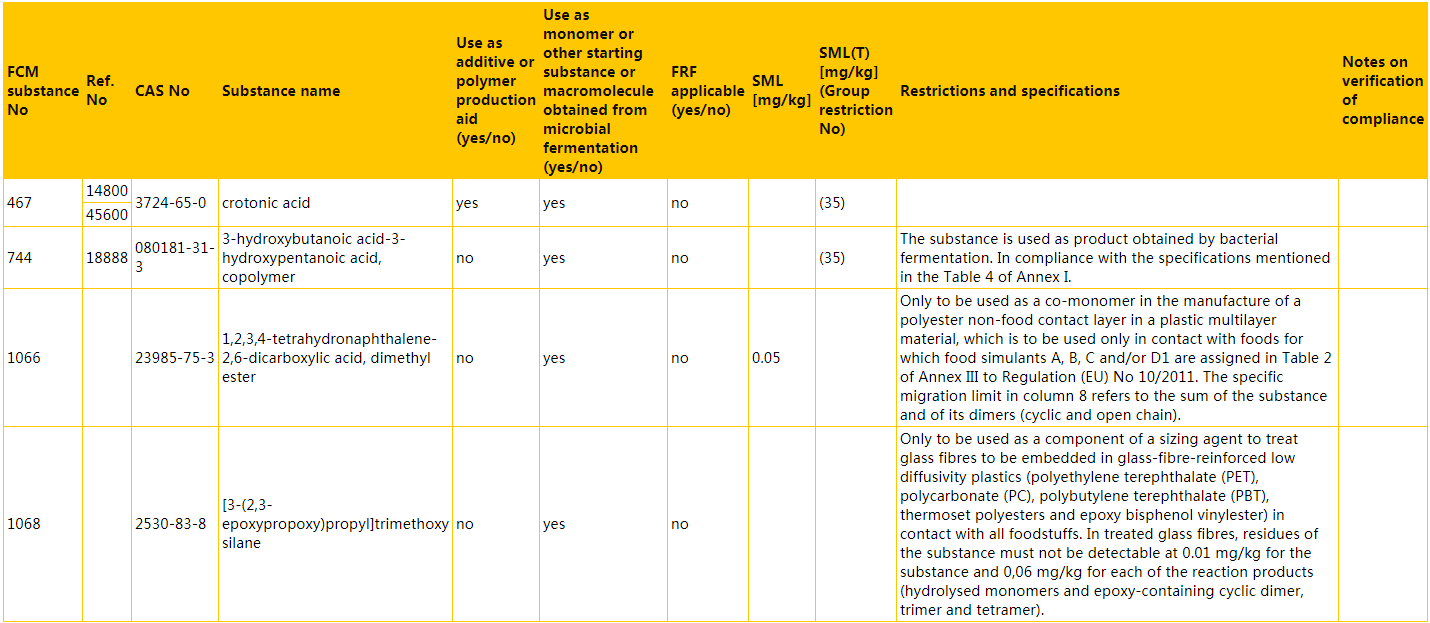
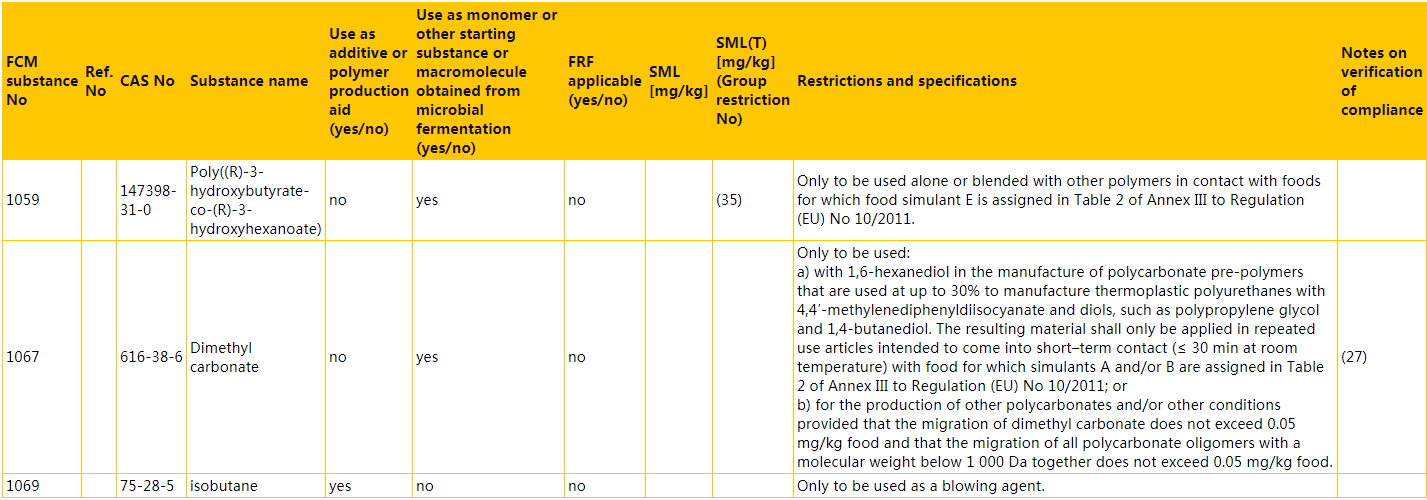
b) the following entries are inserted in numerical order of the FCM substance numbers:
2. Table 2. “Group restriction of substances”
The following entry is added:
|
Group Restriction No
|
FCM substance No
|
SML (T)
[mg/kg]
|
Group restriction specification
|
|
35
|
467
744
1059
|
0.05
|
expressed as crotonic acid
|
3. Table 3. “Notes on verification of compliance”
The following entry is added:
|
Note No
|
Notes on verification of compliance
|
|
(27)
|
When a final material or article containing this substance and produced under conditions other than those described in point a) column 10 of Table 1 is placed on the market, a well described method to determine whether the oligomer migration complies with the restrictions specified in point b) column 10 of Table 1 shall form part of the supporting documentation referred to in Article 16 of Regulation (EU) No 10/2011. This method shall be suitable for use by a competent authority to verify compliance. If an adequate method is publicly available, reference shall be made to that method. If the method requires a calibration sample, a sufficient sample shall be supplied to the competent authority on its request.
|
4. Table 4. “Detailed specification on substances”
The row concerning restriction of the entry concerning substance FCM No 744 is replaced by the following:
|
FCM substance No
|
Detailed specification on the substance
|
|
744
|
Restriction
|
Specific migration limit for crotonic acid is 0.05 mg/kg food
|
B. Annex III Food simulants
1. Table 3. “Food simulant assignment for demonstrating compliance with the overall migration limit” of point 4, the third and fourth rows are replaced by the following:
|
Foods covered
|
Food simulants in which testing shall be performed
|
|
all aqueous and alcoholic foods and milk products with a pH ≥ 4.5
|
food simulant D1
|
|
all aqueous and alcoholic foods and milk products with a pH < 4.5
|
food simulant D1 and food simulant B
|
About Intertek
Intertek is a leading Total Quality Assurance provider to industries worldwide. Our network of more than 1,000 laboratories and offices and more than 100 countries, delivers innovative and bespoke Assurance, Testing, Inspection and Certification solutions for our customers’ operations and supply chains. Intertek Total Quality Assurance expertise, delivered consistently with precision, pace and passion, enabling our customers to power ahead safely.
www.intertek.com.cn
